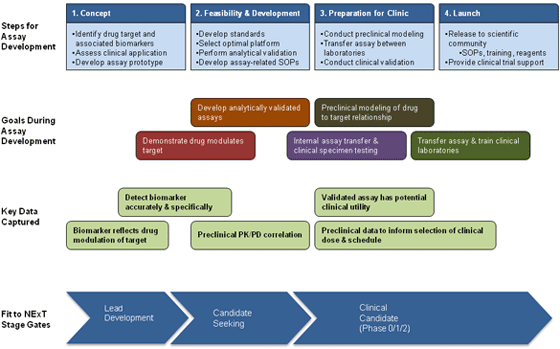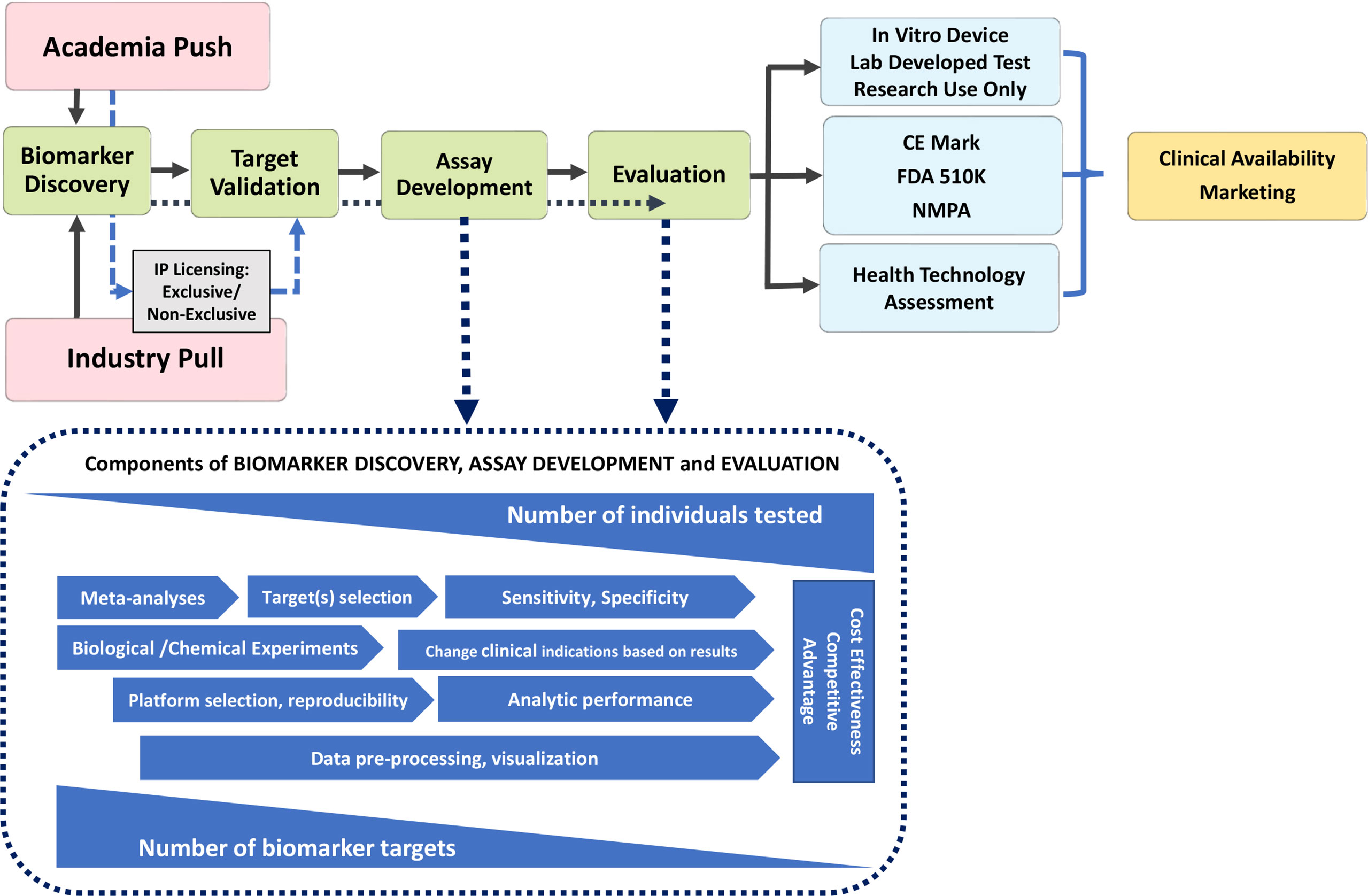
Frontiers | Autoantibody Discovery, Assay Development and Adoption: Death Valley, the Sea of Survival and Beyond | Immunology

Consensus guidelines for the validation of qRT-PCR assays in clinical research by the CardioRNA consortium: Molecular Therapy - Methods & Clinical Development

Assay that Combines Clinical, Radiomic Characteristics with CT Information Shown to Better Distinguish Malignant Early Lung Nodules - ILCN.org (ILCN/WCLC)

Diagnostic assays for patient stratification in clinical trials. A,... | Download Scientific Diagram
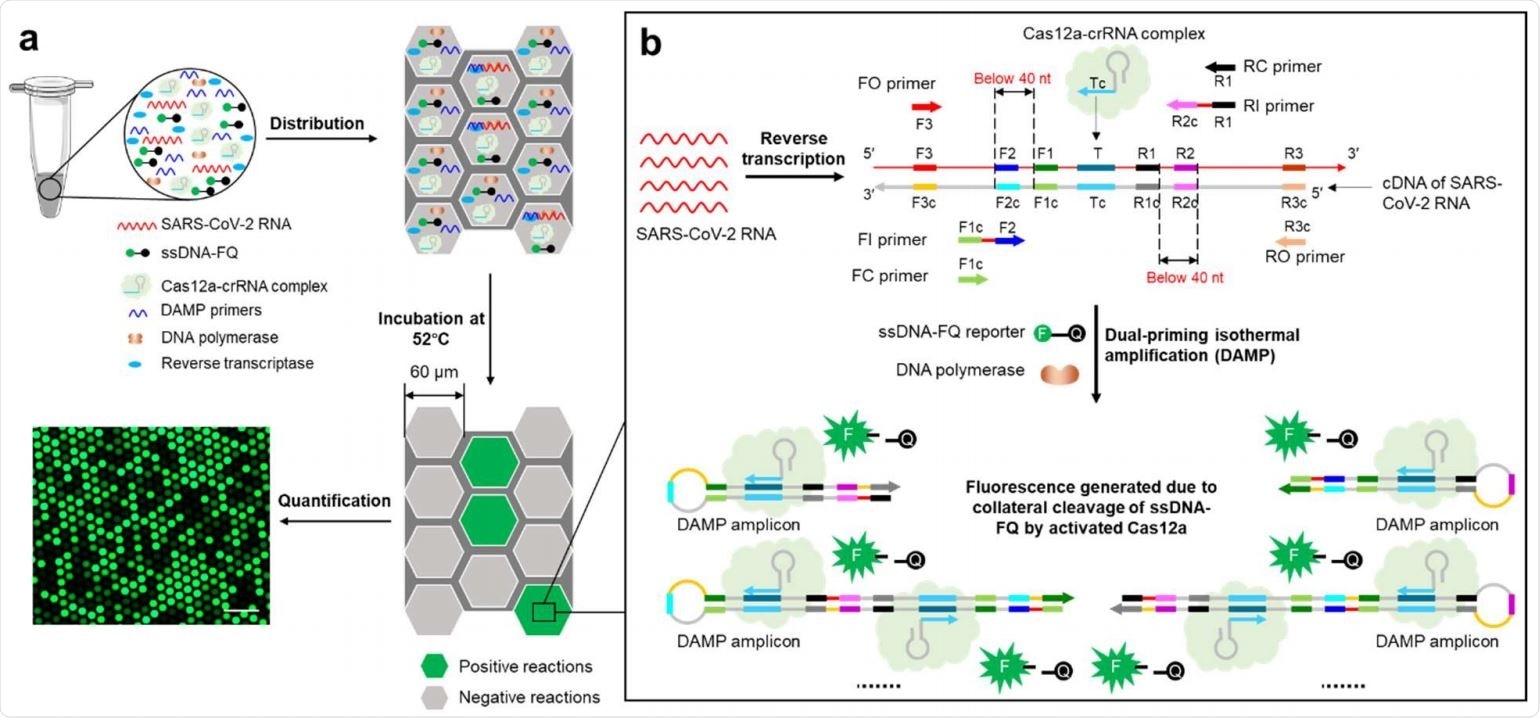
Digital warm-start CRISPR assay enables sensitive quantitative detection of SARS-CoV-2 in clinical samples