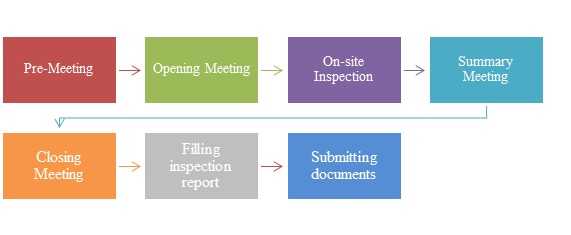
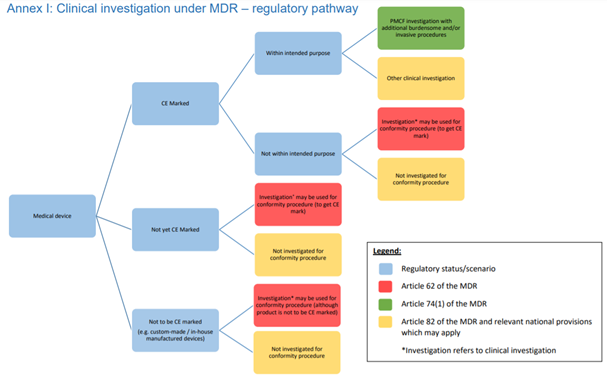
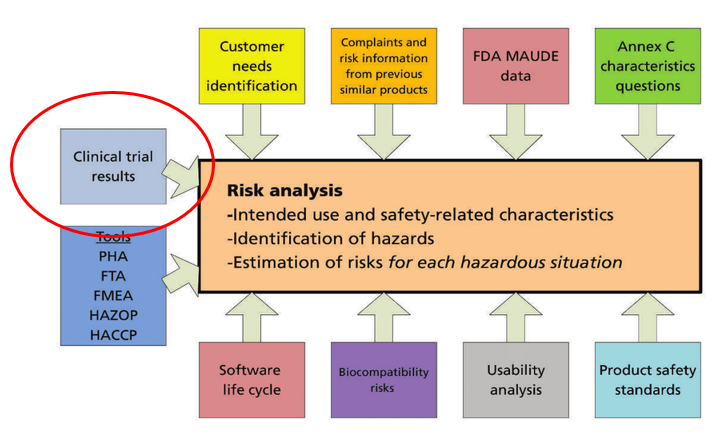
MDR - Medical Device Regulation. Regulation of the EU- European Union on the Clinical Investigation and Sale of Medical Devices Stock Vector - Illustration of healthcare, regulate: 212157525

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention

Improved clinical investigation and evaluation of high-risk medical devices: the rationale and objectives of CORE–MD (Coordinating Research and Evidence for Medical Devices) in: EFORT Open Reviews Volume 6 Issue 10 (2021)
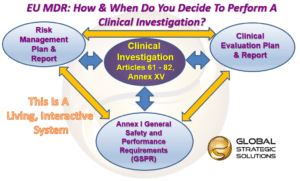
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions

Regulation of Medical Devices and their Clinical Trials Studies in the USA: An Update | Bentham Science