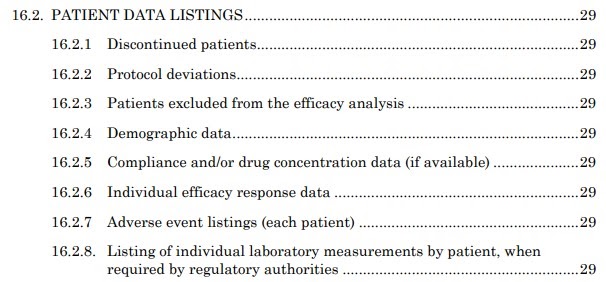
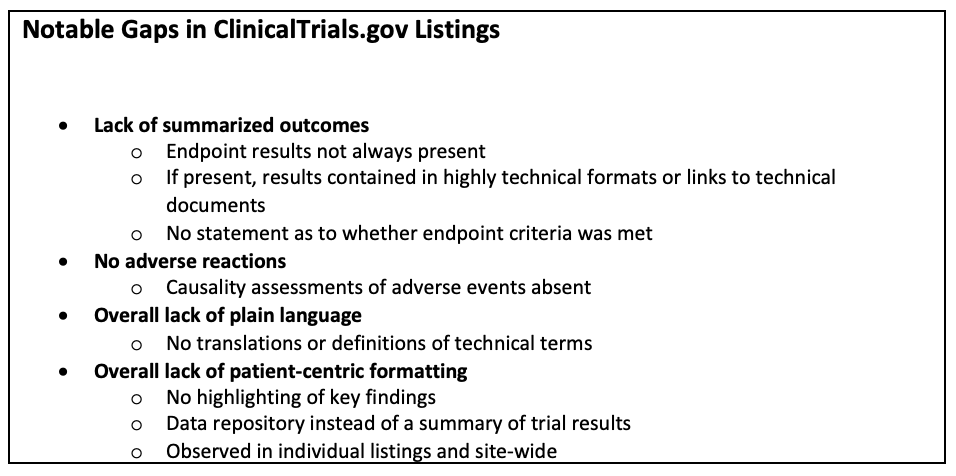
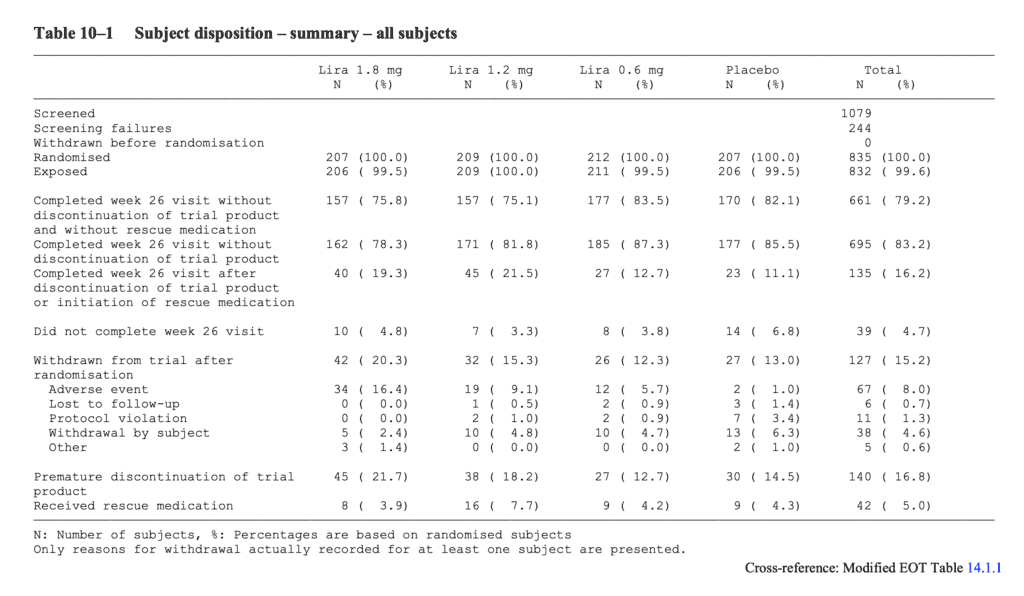
Jack of all Listings, A New Approach for Review of Clinical Data Hardik Panchal, Celgene Corporation, NJ

On Biostatistics and Clinical Trials: Are we moving away from data listings now that the standard data sets (such as CDISC-compliant SDTM and ADaM data sets) are mandated by FDA?

Recruitment of Black Adults into Cardiovascular Disease Trials | Journal of the American Heart Association
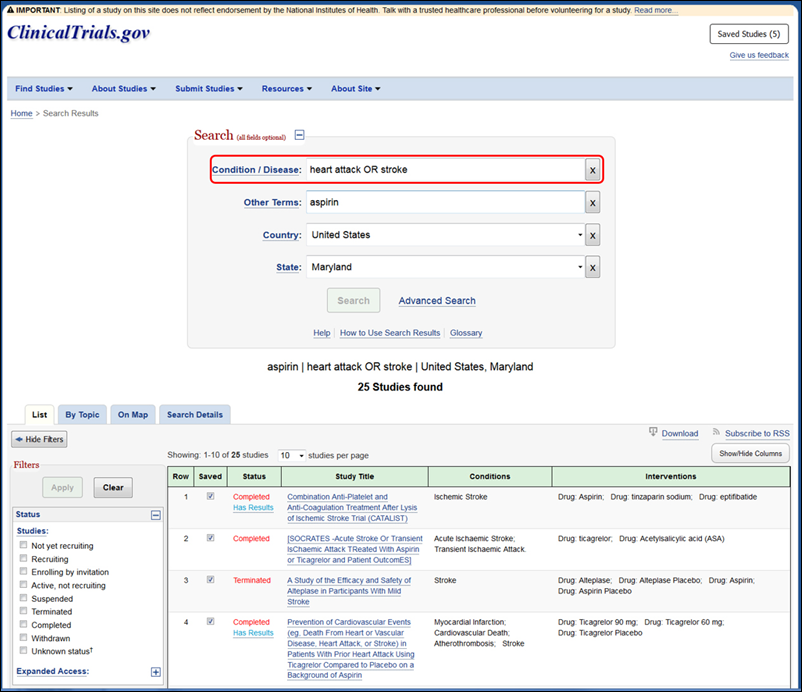
ClinicalTrials.gov: First in a Series of Changes to Improve Usability for Stakeholders. NLM Technical Bulletin. 2017 May–Jun

SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch
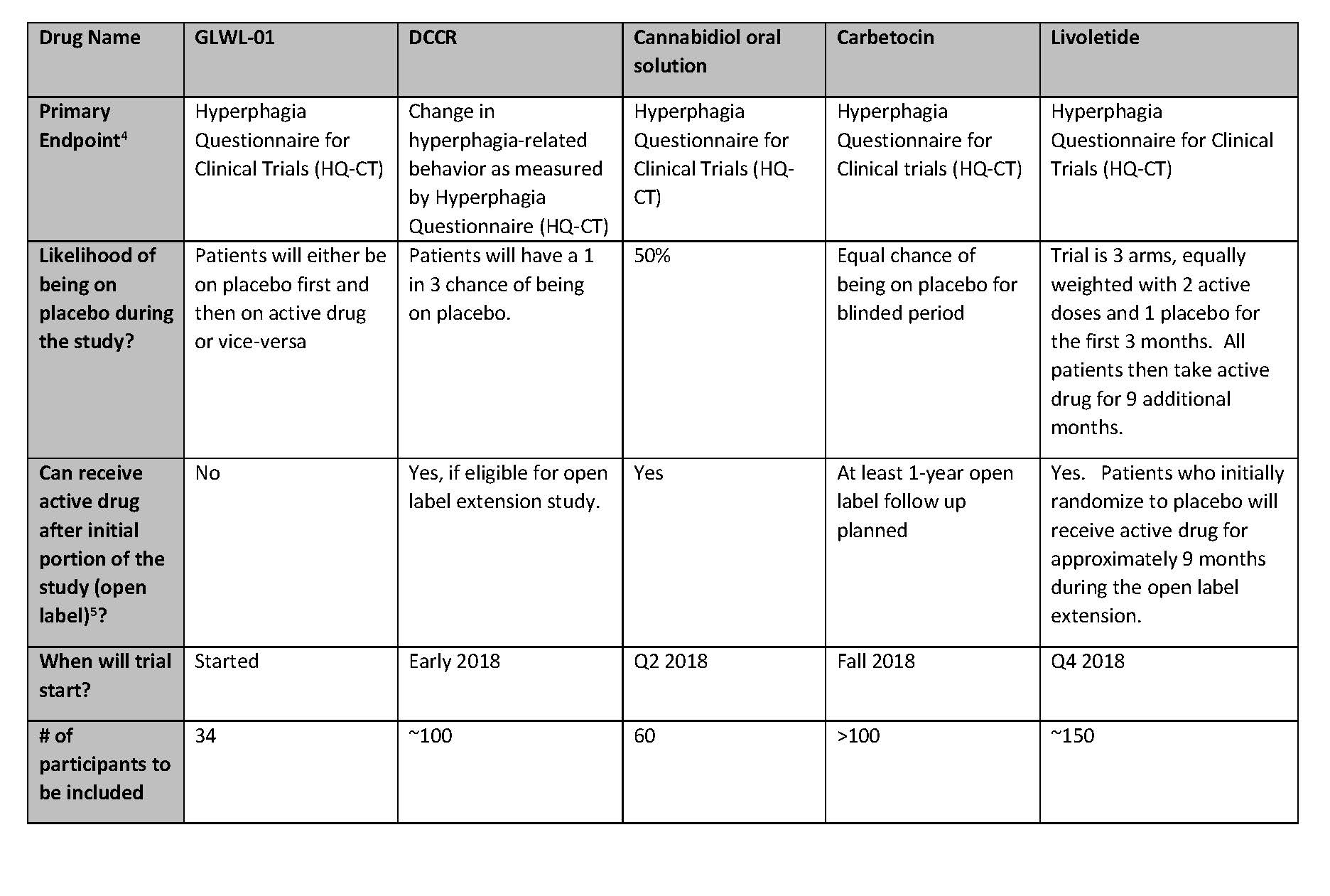
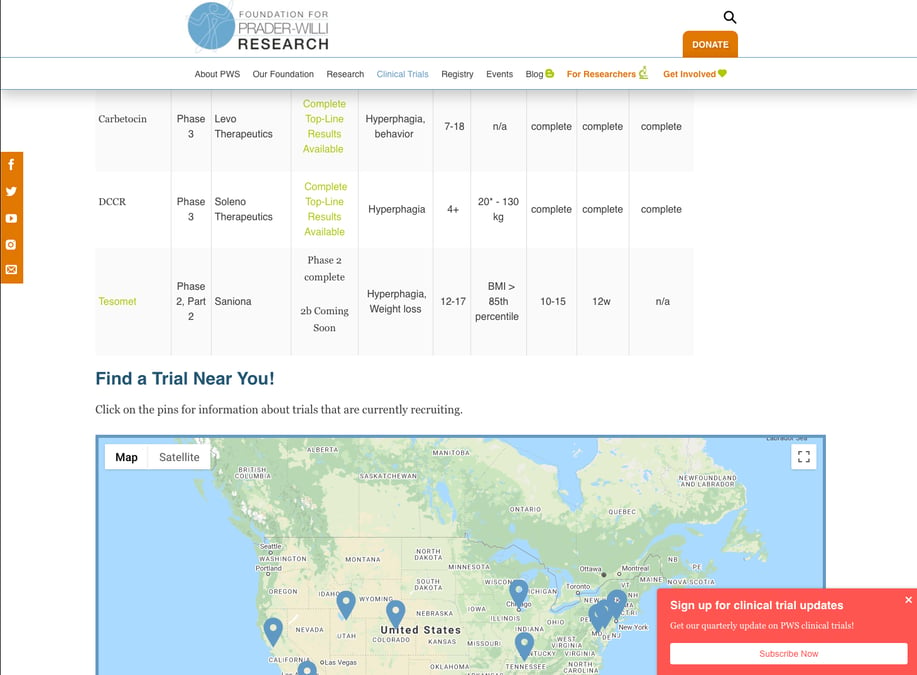
Summary Of Active Clinical Trials For Prader-Willi Syndrome Hyperphagia - Prader-Willi Syndrome Association | USA
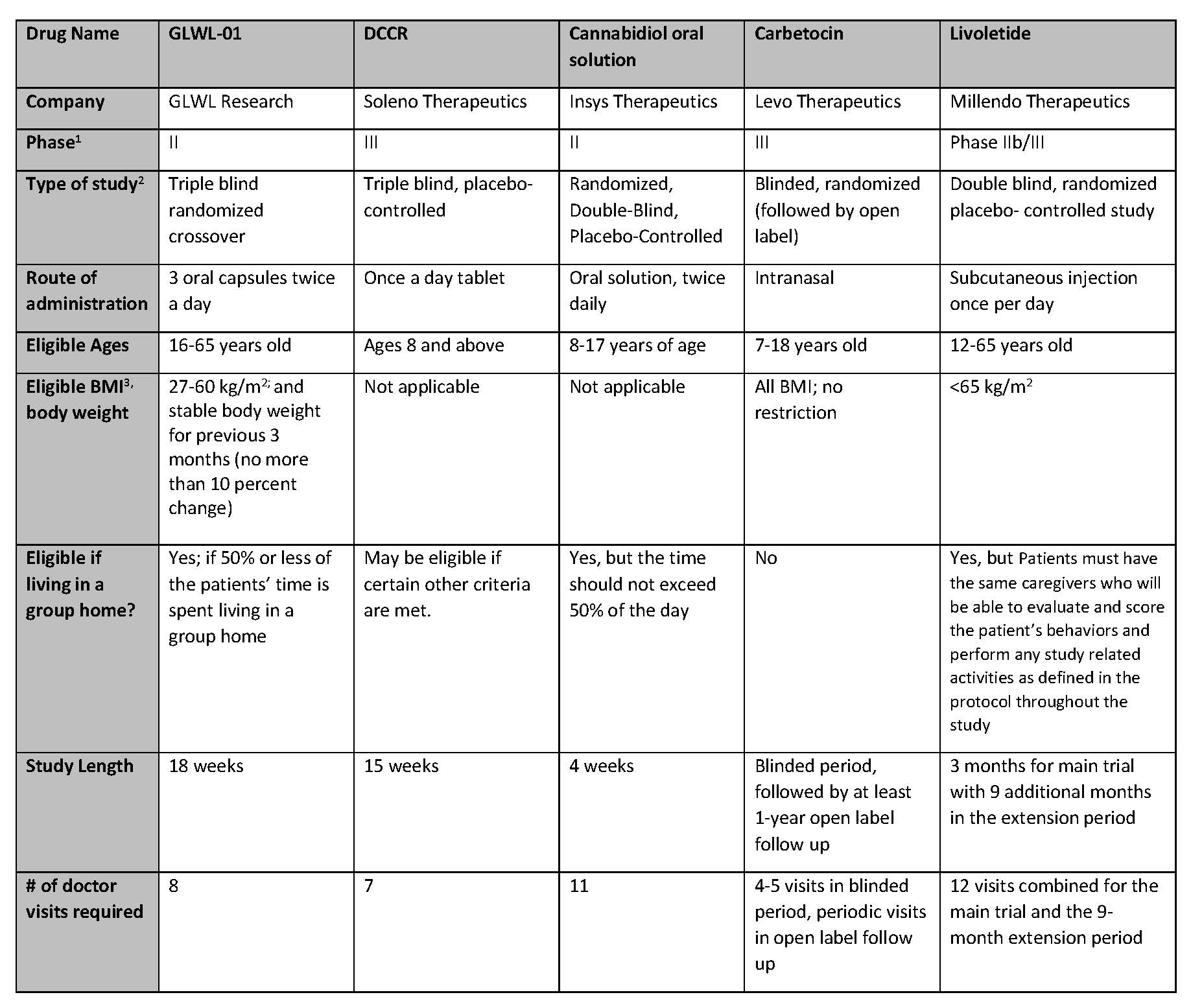
Summary Of Active Clinical Trials For Prader-Willi Syndrome Hyperphagia - Prader-Willi Syndrome Association | USA