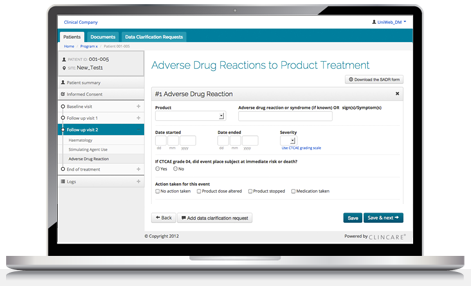
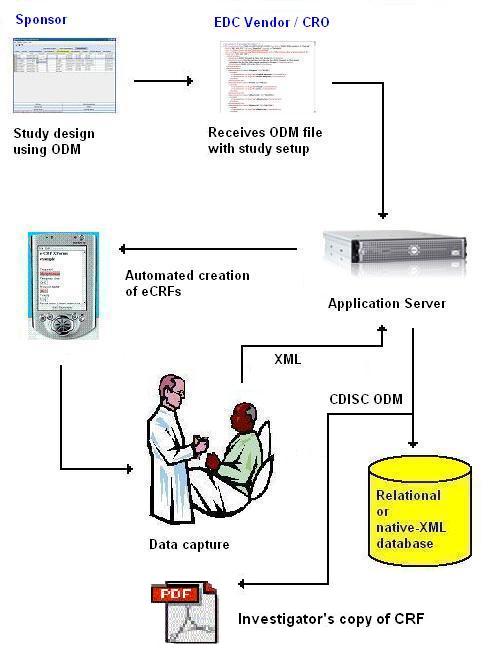
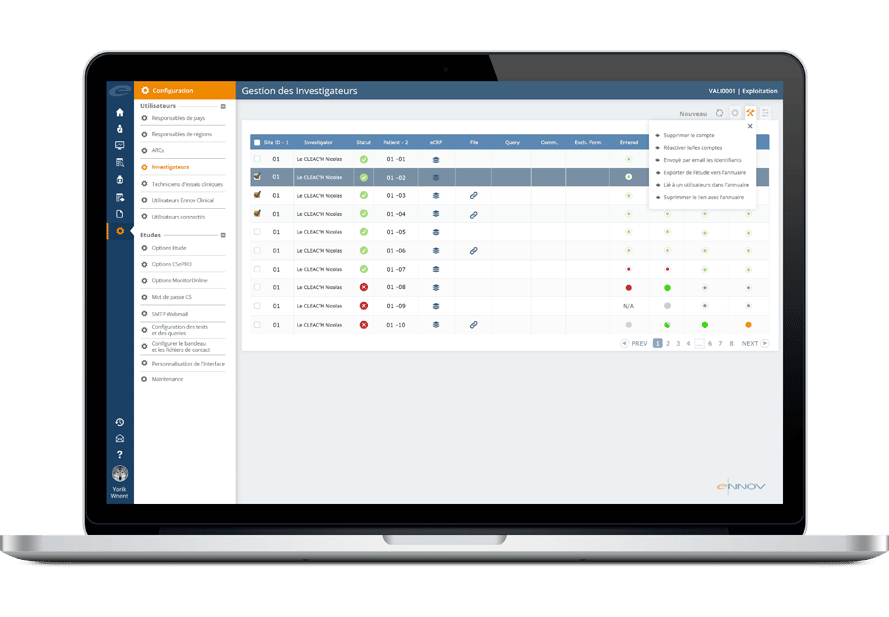
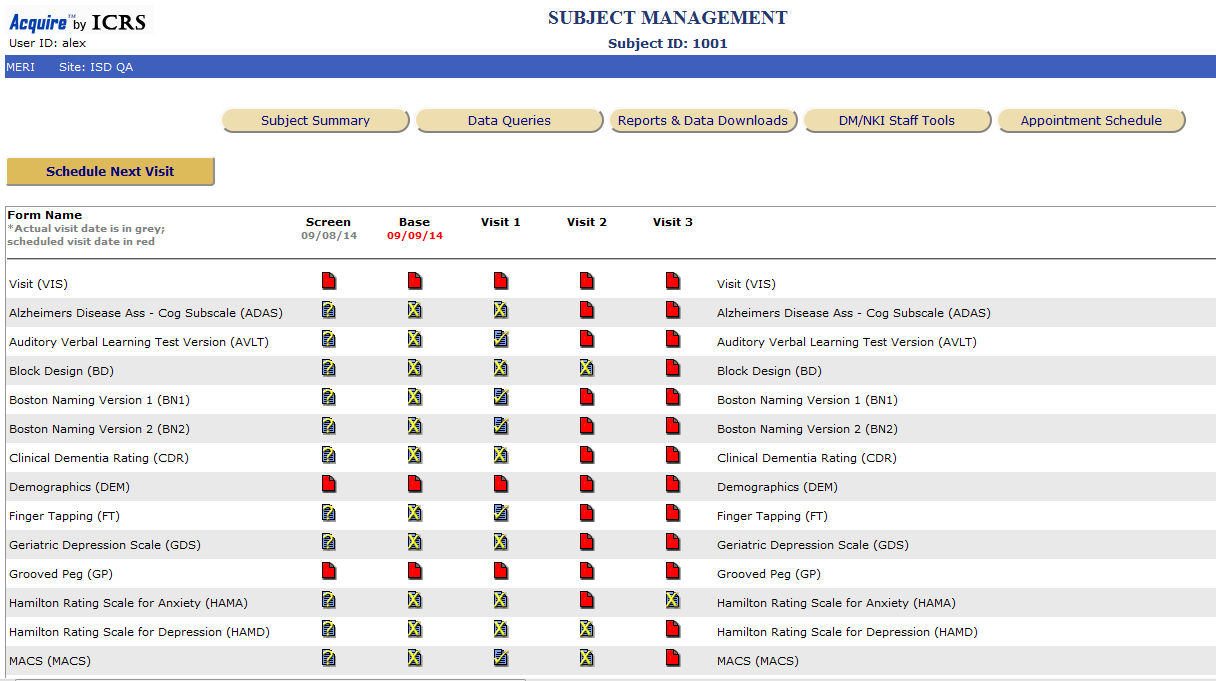
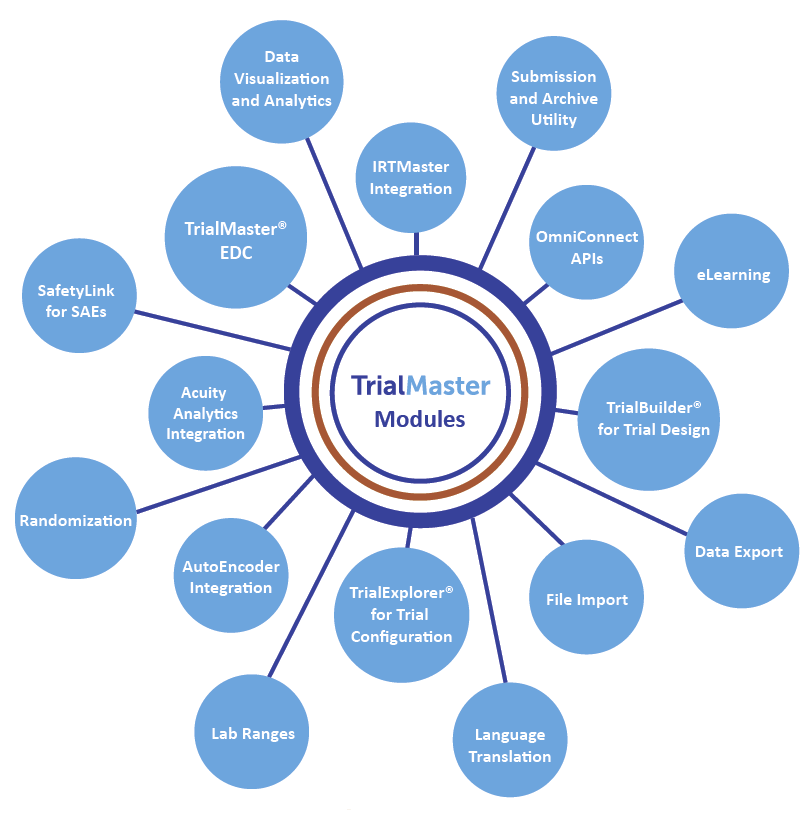
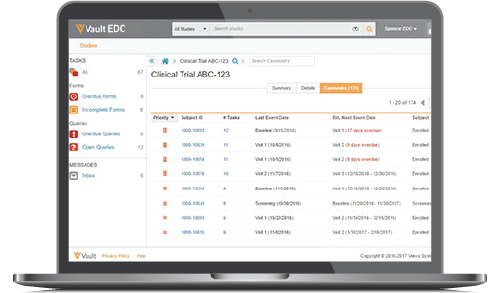
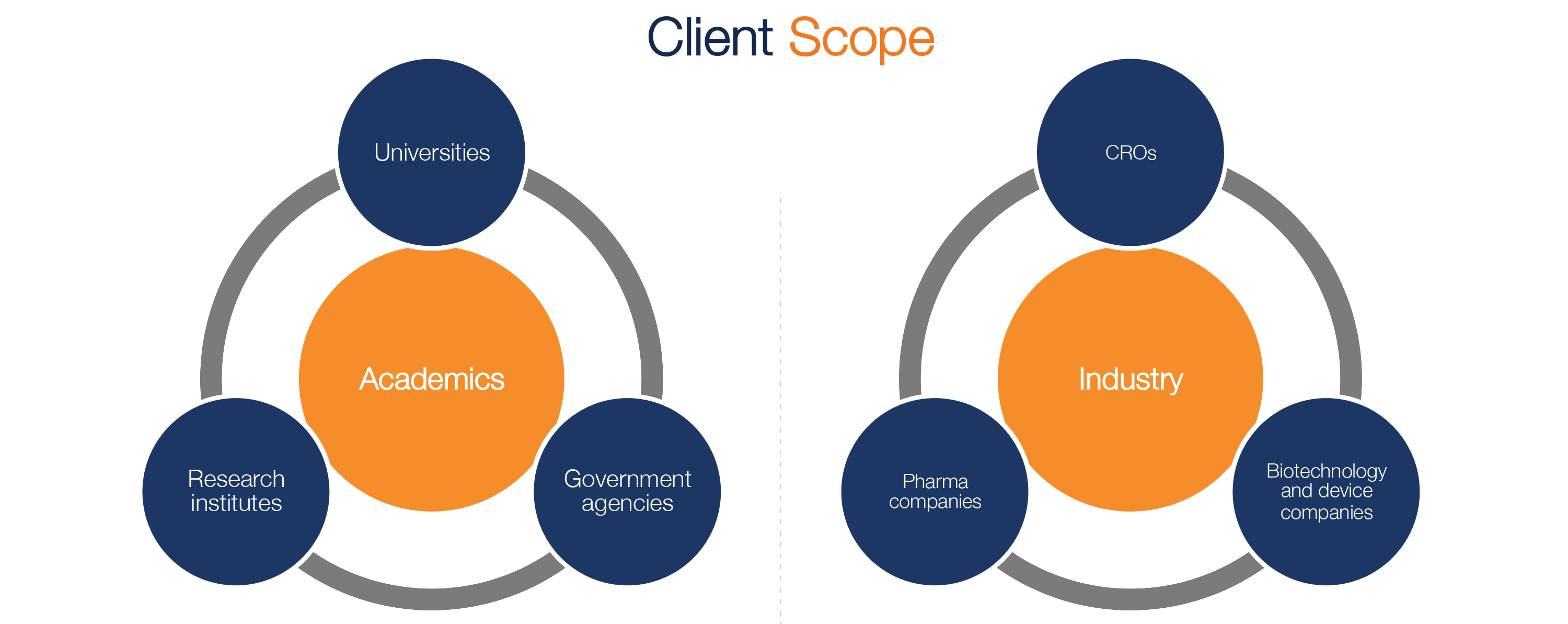
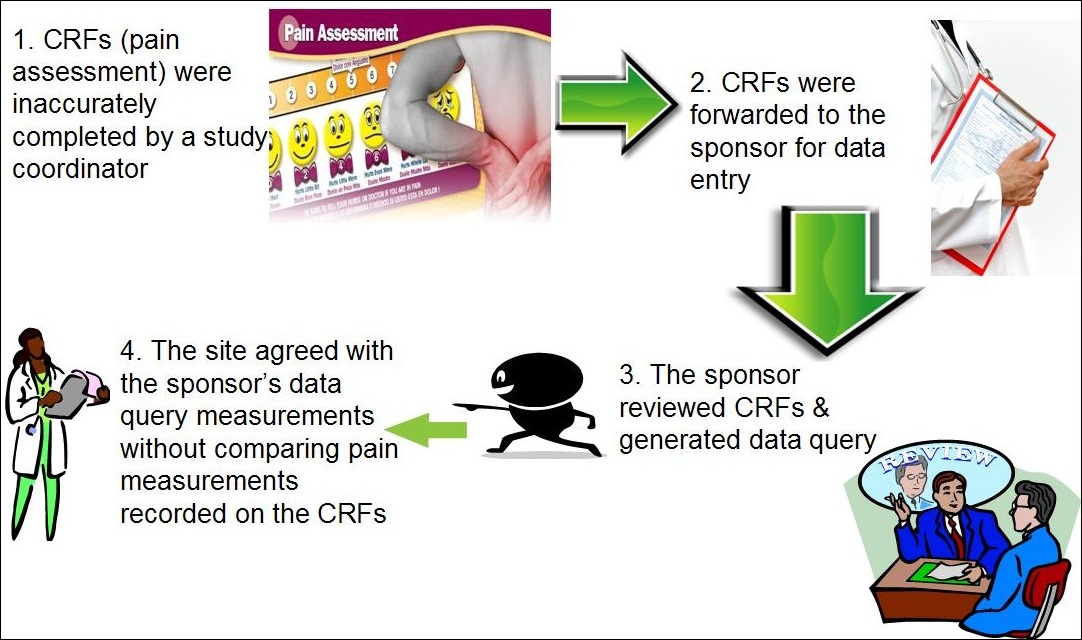
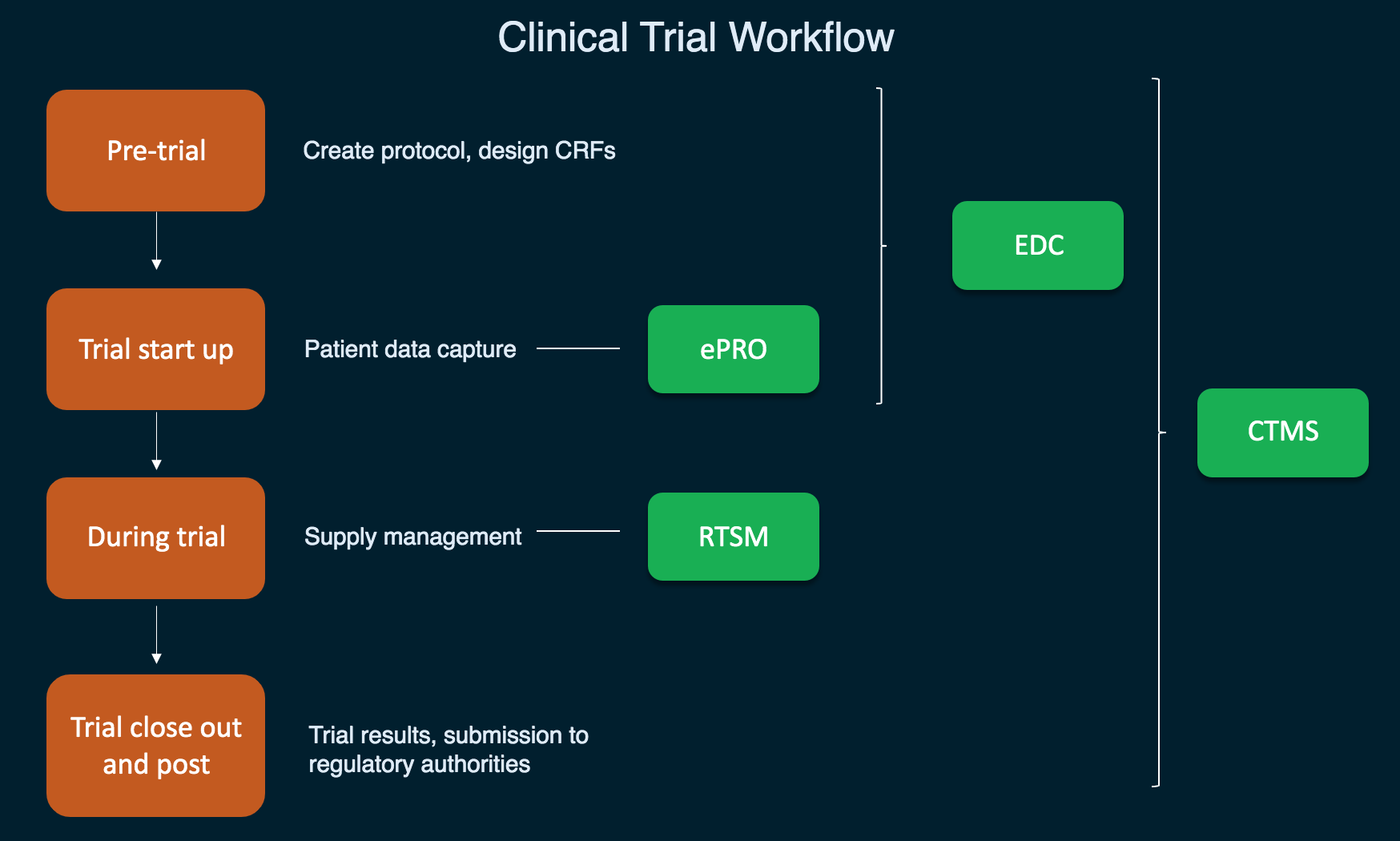
Pharma and biotecht Phase (I – II – III and IV) Clinical Trials | Electronic Data Capture (EDC) as a useful – ResearchManager
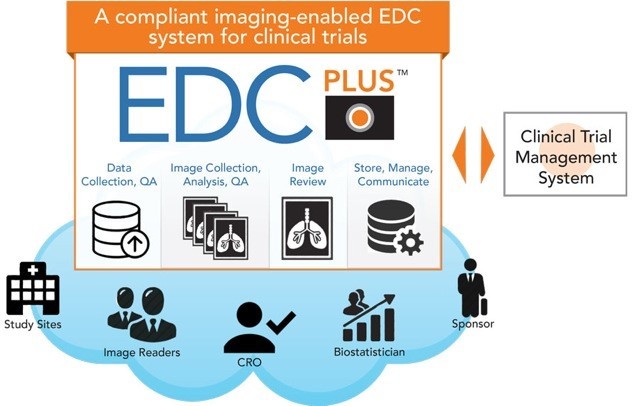
ImageIQ Releases EDCplus Technology, an Imaging-enabled Electronic Data Capture System for Clinical Trials | Business Wire