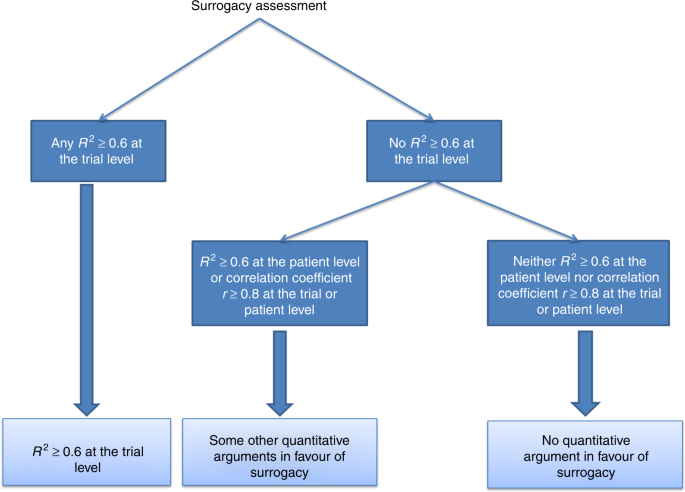
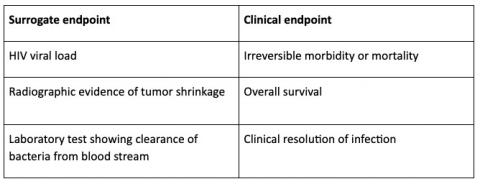
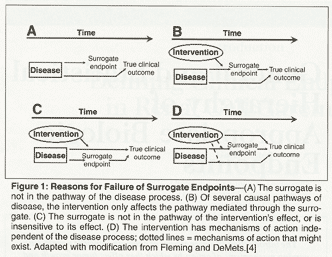
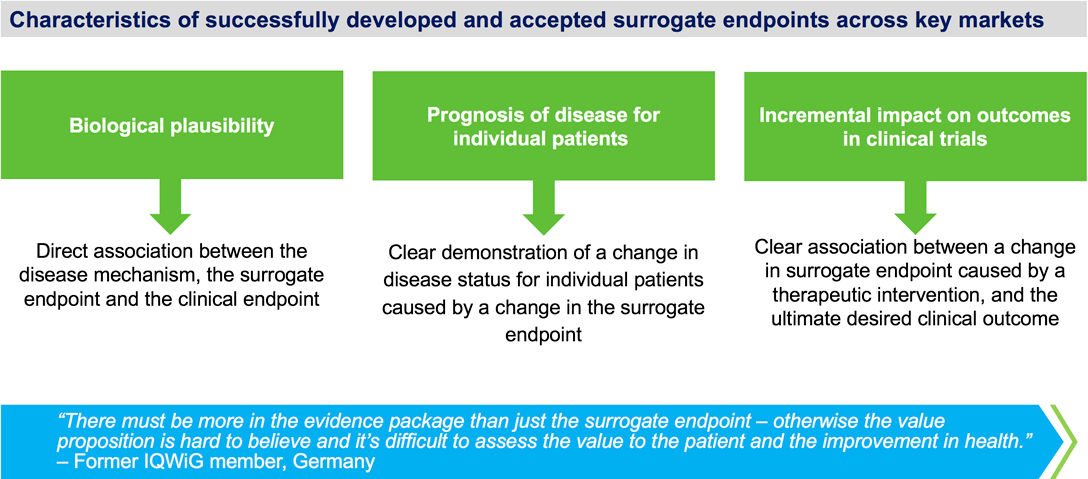
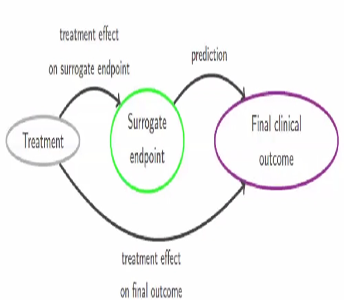
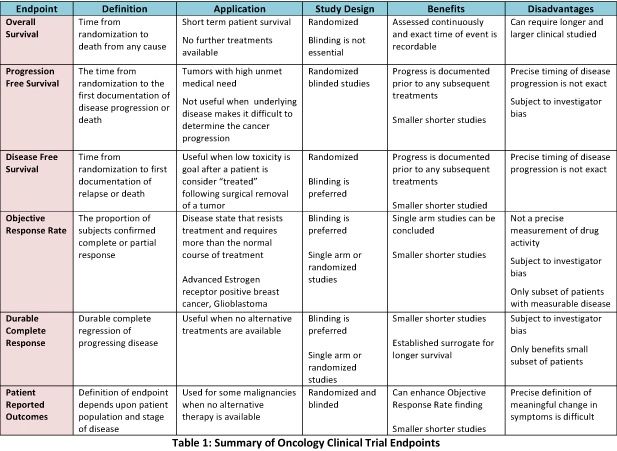
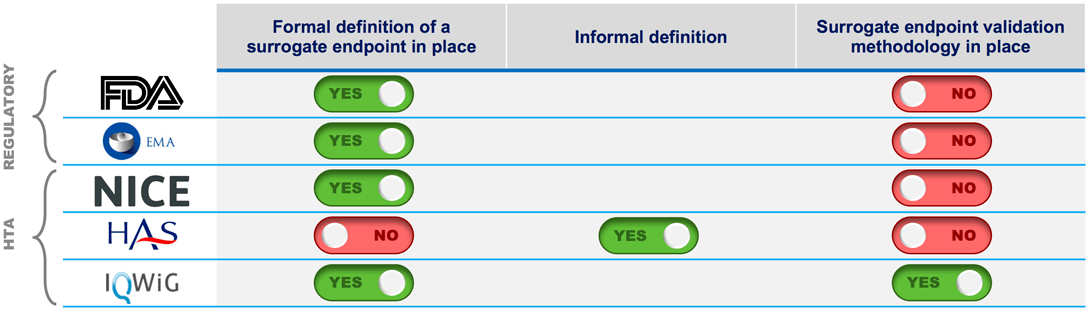
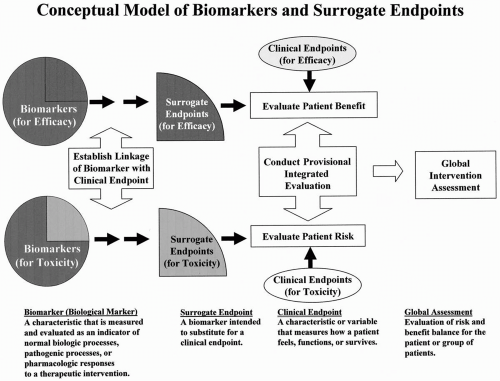
Time to Review the Role of Surrogate End Points in Health Policy: State of the Art and the Way Forward - Value in Health
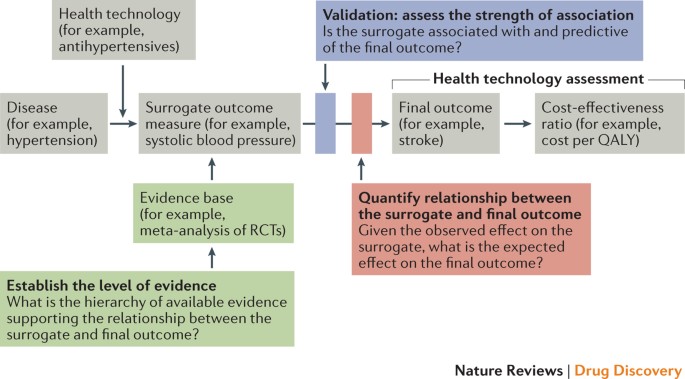
Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework | Nature Reviews Drug Discovery

Surrogate Endpoints in Infectious Diseases Trials: FDA Perspective Surrogate Endpoints in Infectious Diseases Trials: FDA Perspective John H. Powers, MD. - ppt download
![PDF] Foreword: Biomarkers and Surrogate Endpoints in Ophthalmic Clinical Research. | Semantic Scholar PDF] Foreword: Biomarkers and Surrogate Endpoints in Ophthalmic Clinical Research. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/112d32b4103ed729020697b7fd92b35091b6fc6e/2-Figure1-1.png)
PDF] Foreword: Biomarkers and Surrogate Endpoints in Ophthalmic Clinical Research. | Semantic Scholar
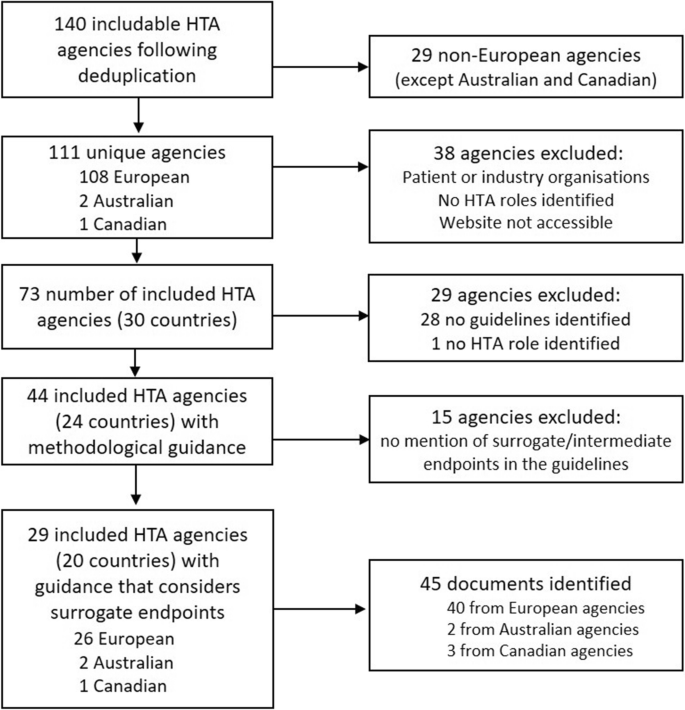
Surrogate Endpoints in Health Technology Assessment: An International Review of Methodological Guidelines | SpringerLink