![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/9-Figure4-1.png)
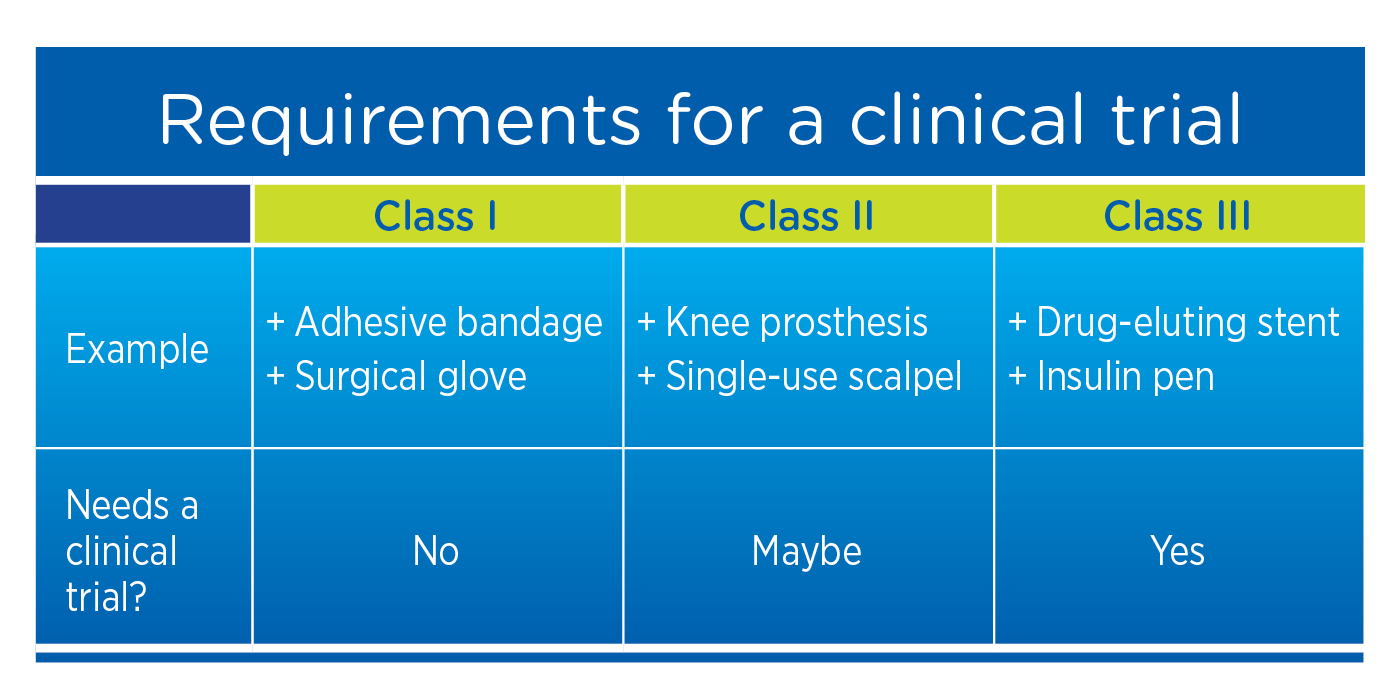
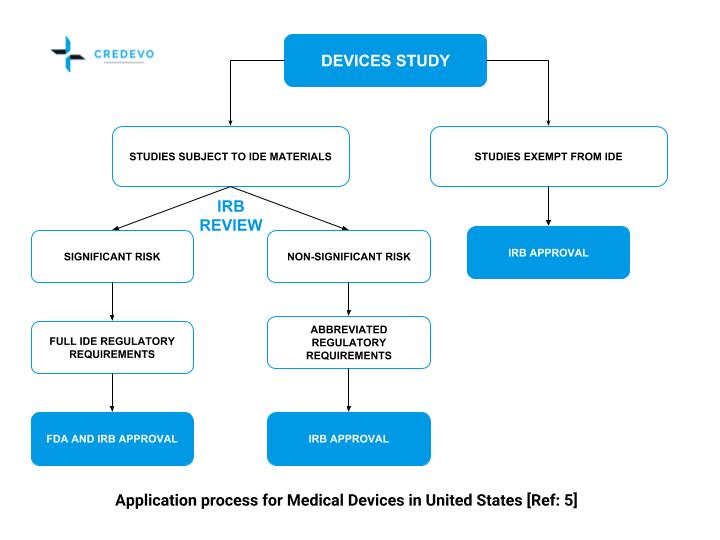
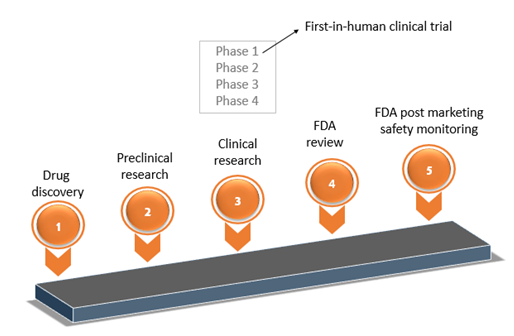
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

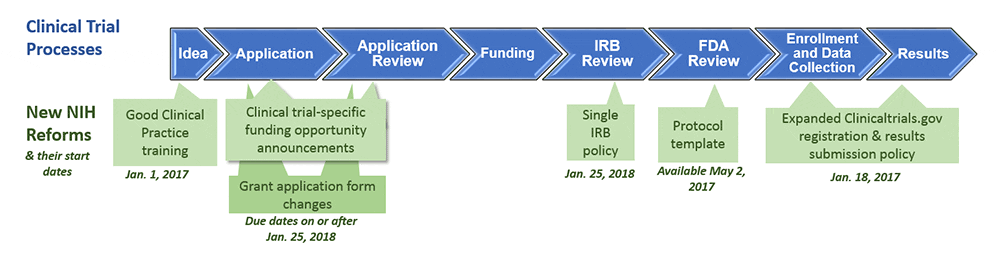
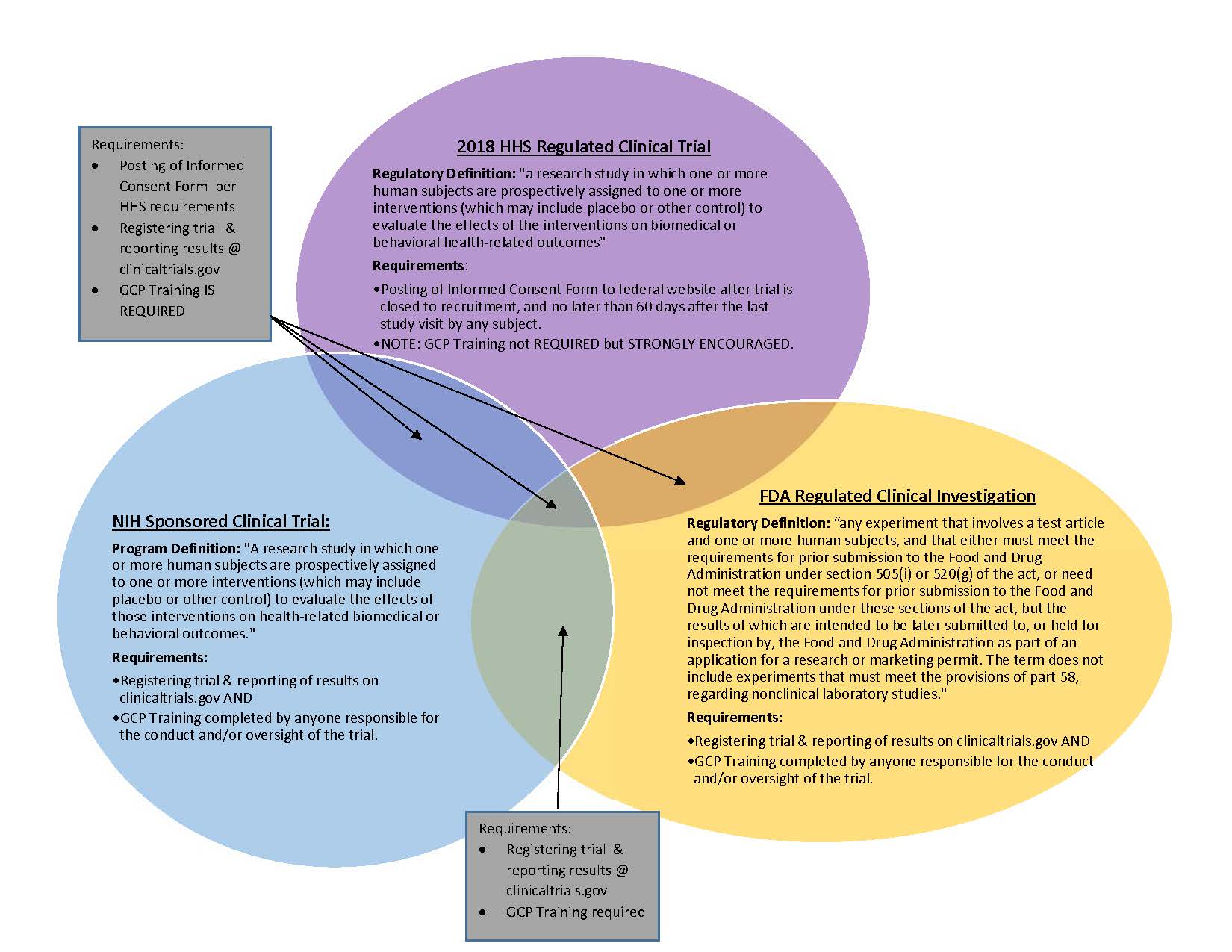
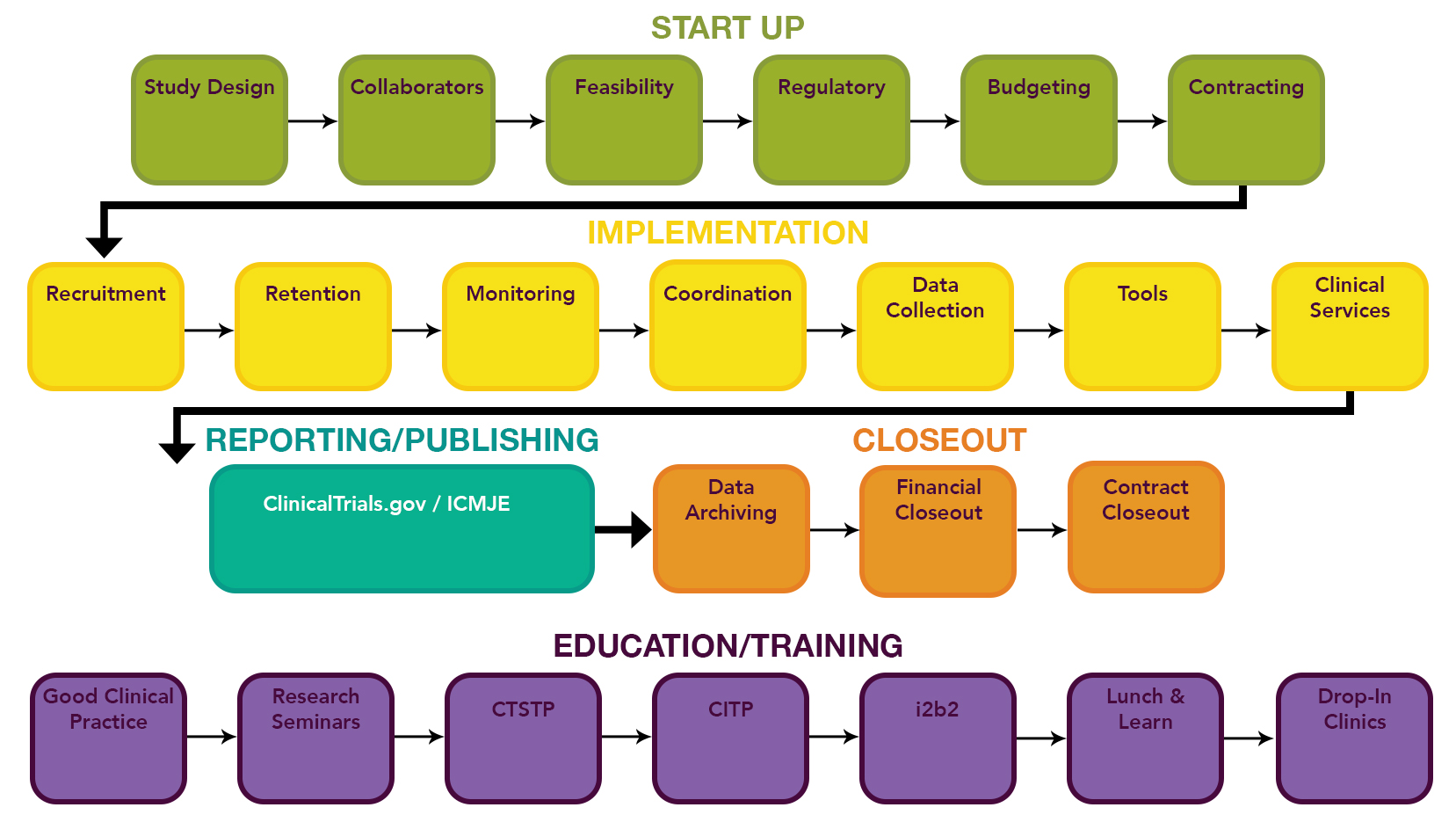
Atlanta Pediatric Research | NIH Requirements for Human Subject Research | Clinical Research Resources | Research Resources | Research | Emory + Children's + GT | Atlanta Pediatric Research Alliance

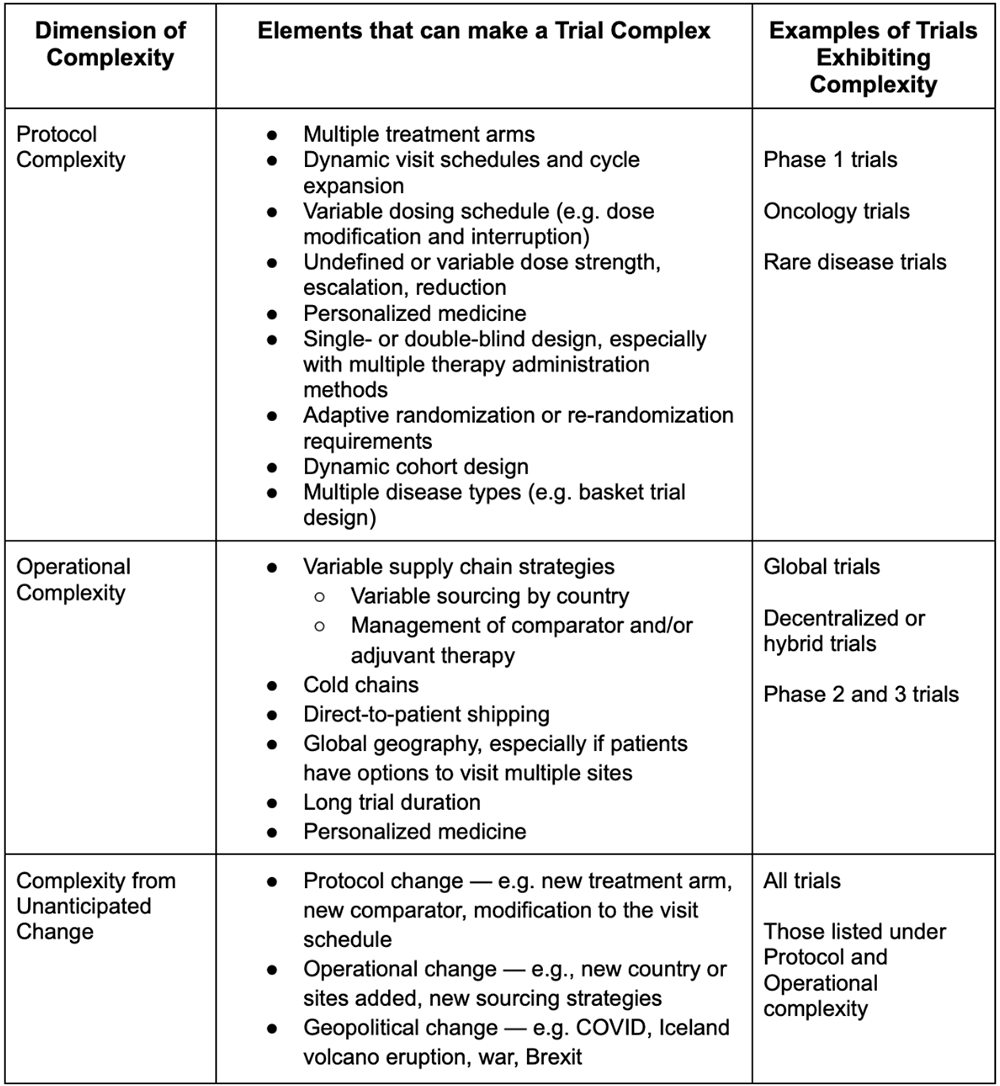
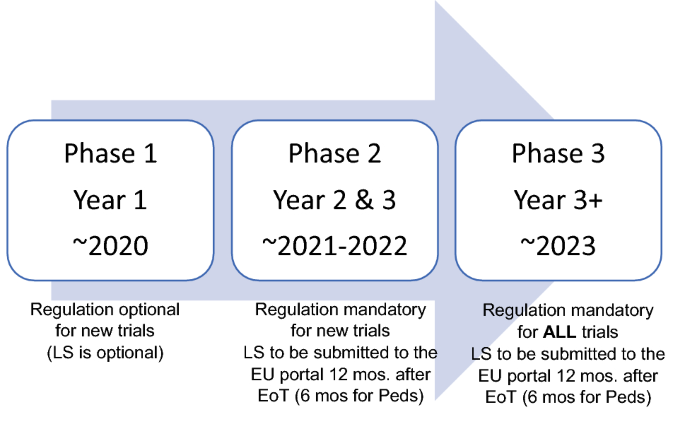
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS